Metastatic Squamous Cell Carcinoma to the Parotid Gland – A Comprehensive Analysis
Rawan Azzam1*, Rani Hammoud1, Rajen Goyal2, Majid Al Abdulla1
1Otolaryngology Department, Ambulatory care center, Hamad medical Corporation (HMC), Doha – Qatar
2Histopathology Department, Hamad medical Corporation (HMC), Doha – Qatar
*Corresponding author: Rawan Azzam, MD, Ambulatory care center, HMC, Doha-Qatar 3050. Tel: +97455320145.
Citation: Azzam R, Hammoud R, Goyal R, Al Abdulla M (2024) Metastatic Squamous Cell Carcinoma to the Parotid Gland – A Comprehensive Analysis. Annal Cas Rep Rev: ACRR-405.
Received Date: 24 July, 2024; Accepted Date: 30 July, 2024; Published Date: 06 August, 2024
Abstract
Although most of the malignant neoplasms of salivary glands affect the minor and sublingual salivary glands, about 20% of these tumors affect the parotid gland. However, primary squamous cell carcinoma in the parotid gland is rare, estimated to be less than 4% [1]. It is even rarer for squamous cell carcinoma to spread to the parotid gland, accounting for only 1-3% of all parotid cancers. This report is about a 64-year-old man who is known to be on immunosuppressants due to history of kidney transplant, had history of cutaneous squamous cell carcinoma in the crown of scalp that was excised with narrow margins about one year ago outside Qatar. Patient started to notice a right sided parotid lump that grows over one month. A fine needle aspiration of that swelling reveled a poorly differentiated squamous cell carcinoma. This report examines the patient’s presentation of symptoms, the diagnostic process, the treatment administered, and the significance of considering cancer metastasis in the diagnosis of parotid masses, particularly in individuals with compromised immune systems.
Keywords: Squamous cell carcinoma, parotid gland, metastasis, immunosuppression, parotid malignancy, FNA, MRI, PET CT, adjuvant therapy.
Introduction
Squamous cell carcinoma (SCC) is an aggressive epithelial malignancy arising from uncontrolled proliferation of squamous cells. These cells typically line various body surfaces, including the skin, oral cavity, and respiratory tract. SCC accounts for a significant portion of all non-melanoma skin cancers and can also develop in mucosal linings [3]. While the vast majority of SCC cases are primary, meaning they originate in the location where they are first detected, a small percentage can metastasize, or spread, to distant sites in the body [5].
One common site for benign tumors is the parotid gland, belonging to the three major salivary glands located on both sides of the face. However, primary SCC of the parotid is a relatively rare entity [2]. On the other hand, these are of low frequency of occurrence (1-3%) among all parotid malignancies in the parotid gland and rarely metastasize to other organs [4].
This case report presents a comprehensive analysis of a patient who developed metastatic SCC to the parotid gland. This report tries to detail his presentation, workup, diagnosis, and treatment with a view to providing information added to better understanding of this rather under-recognized entity, stressing the importance of considering metastasis in the differential diagnosis for parotid masses, more so in a patient with known malignancy and compromised immune function.
Case Presentation
A 64-year-old gentleman who had been previously diagnosed with end stage kidney disease secondary to autosomal dominant polycystic kidney disease (ADPKD), underwent renal transplantation in 2012 and was kept on maintenance immunosuppressive medications.
He presented with a new right parotid lump. He reported noticing the lump in his right parotid gland approximately one month prior seeking medical attention. There were no other added localizing symptoms, such as facial pain or alteration in swallowing or change in facial sensation.
Further history on the past medical background of the patient showed that he had a past medical burden. He had an interesting history of multiple basal-cell carcinomas (BCCs), which are yet another type of skin cancer that arises from uncontrolled growth of basal cells, located deeper in the epidermis than SCC. Although these basal-cell carcinomas were excised in his home country, along with wide excision of a large SCC on his scalp roughly a year before presenting with the parotid lump, there were multiple scalp actinic keratosis lesions confirmed with punch biopsies done by the dermatology team in Qatar.
Notably, the previous scalp SCC excision reported having narrow margins, potentially increasing the risk of local recurrence or distant metastasis. Patient was advised for wider excision and revision of margins by the surgical team in his home country, but he didn’t do it. Patient was prescribed with immunosuppressant medications (mycophenolate mofetil, tacrolimus, prednisolone), antiplatelet (Aspirin), heart health medications (Atorvastatin, Lisinopril, Lansoprazole), antidepressant (Amitriptyline) and vitamins (D3 and B). No significant findings regarding tobacco use, alcohol consumption, or occupational exposures known to increase SCC risk was found in patient social history. No significant findings of familial predisposition to SCC or other malignancies were found in patient family history.
Examination
In physical examination all vital signs were within normal limit. Patient was well nourished and alert. Head and neck examination showed a three cm firm slightly tender non-mobile mass in the right parotid gland. There was no palpable cervical lymph nodes. Facial nerve function was normal. Multiple scabs on the scalp were present because of recent biopsies. Also, there was well-healed scar from the previous scalp SCC excision.
Differential diagnosis
Multiple differentials were made including primary parotid SCC (less likely due to history of scalp SCC), Warthin’s tumor (benign parotid tumor), pleomorphic adenoma (benign parotid tumor) and metastatic carcinoma to the parotid gland (most likely due to history of scalp SCC).
Investigations
1. Radiology
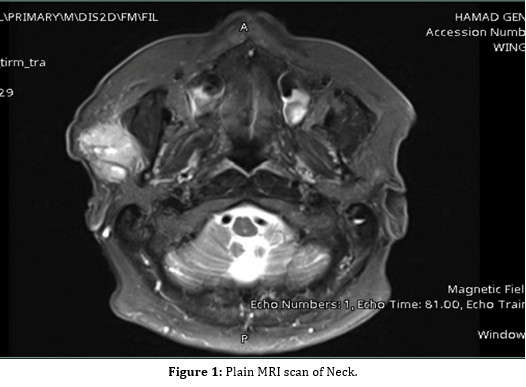
Magnetic resonance imaging (MRI) of the neck was performed to evaluate for local invasion of the parotid gland and potential metastasis to lymph nodes in the neck. It showed two right parotid space lesions adjacent to each other showing heterogenous T2 signal with diffusion restriction and hypointense T1 signal intensity, the larger lesion measures 25 x 13 x 22.5 mm in AP, TR and CC dimensions and the smaller lesion measures around 9 x 16 x 18 mm (Figure 1). Both lesions were highly suspicious for nodal metastasis. No other similar lesions noted, and the rest of the neck soft tissues appear unremarkable
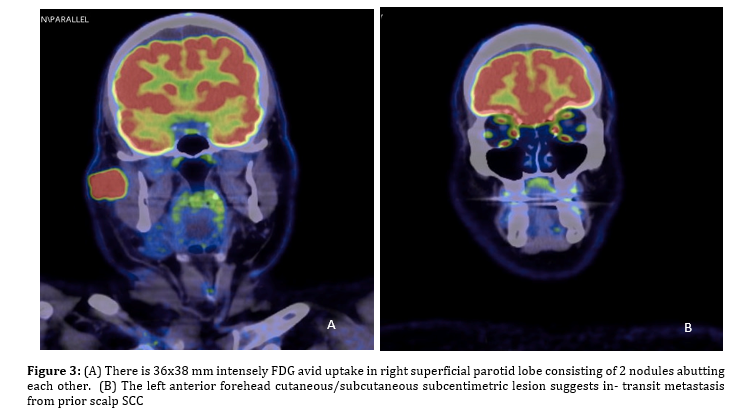
A PET CT scan was done and had demonstrated increased intensity of metabolic activity of the right parotid mass, which is considered to be sign of malignant lesion. Equally important is that there were no distant metastatic foci identified on the PET CT scan (Figure 3).
2. Pathology
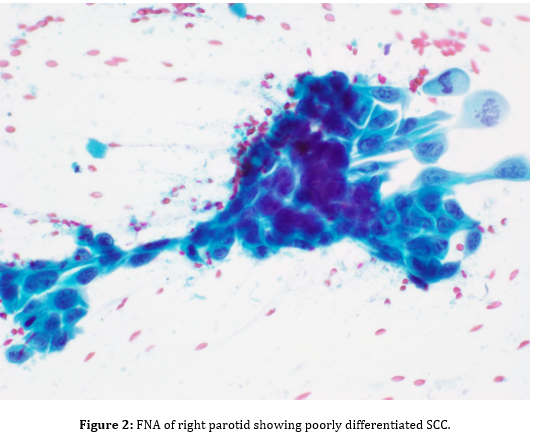
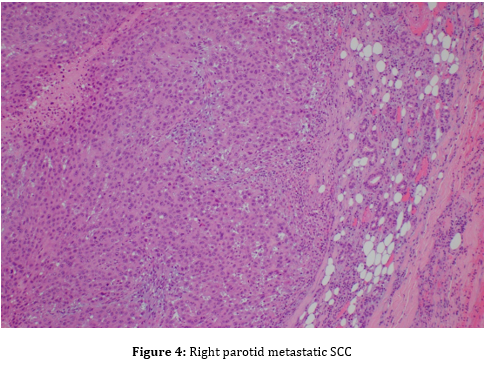
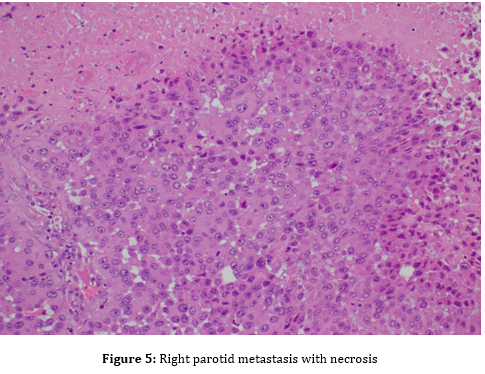
Fine-needle aspiration (FNA) of the parotid mass was ordered to obtain cells for microscopic analysis. FNA showed malignant cells, consistent with a poorly differentiated squamous cell carcinoma (Figure 2). Microscopic examination of the parotid mass post total parotidectomy showed a poorly differentiated carcinoma with squamous features, most consistent with metastatic squamous cell carcinoma (Figure 4), likely cutaneous in origin. Tumor mass was three cm in greatest dimension, obliterated parotid tissue with invasion into surrounding adipose and skeletal muscle tissues
Treatment
Based on the findings of the diagnostic work-up, the patient underwent surgical resection of the parotid mass, in form of right total parotidectomy with a right selective neck dissection (level II), along with revision of margins of the scalp SCC, which all came back negative for malignancy. Due to the invasion of lympho-vascular structures, and after discussion in a multidisciplinary team meeting, a decision was made to send the patient for an adjuvant radiotherapy to the neck in Qatar, but patient preferred to receive it in his home country.
Discussion
The history of SCC on the patient’s scalp made us really suspect that the cancer could have spread to his parotid gland, even if this doesn’t happen much.
Subsequently, the case illustrates the impact of a weakened immune system on the proliferation of cancer and its metastasis. Medications to suppress the immune response are frequently administered post-organ transplantation to prevent rejection. But these drugs might also lower the body’s guard against cancer cells. If anything, his weak immune system, as a result of a kidney transplant, could have probably given the cancer an upper hand in growing and spreading from the scalp to the parotid gland.
This is an approach that could be used to evaluate a parotid mass when performing diagnostic steps. The FNA, also an outpatient procedure, was a minimally invasive technique through which to get a sample of cells to look at microscopically. It was opted to proceed for further neck magnetic resonance imaging (MRI). The MRI scan provides very good anatomic information about the extent and local invasion of the parotid mass with surrounding structures, including lymph nodes.
The procedure entailed Fine Needle Aspiration, which was a way of having the fine needle enter the parotid mass, aspirating a tiny number of cells, and smearing them on a glass slide. It is then examined under an optical microscope by a pathologist. The results showed malignant cells, which were in keeping with poorly differentiated carcinoma. This test could not make an outright comment as to whether it was primary or metastatic SCC, but they are of value because they indicated malignancy.
After FNA, an MRI scan of the neck was performed to obtain detailed images of the parotid gland, surrounding tissues, and lymph nodes in the neck. Additional investigations were carried out for the diagnosis purposes. A Positron Emission Tomography-Computed Tomography (PET CT) scan was done to further look for the presence and extent of metastatic disease. The PET does so by detecting radioactive tracers that are within the body to find tissues that have metabolically activated.
This was followed by a consultation with a nephrologist to assess the fitness of this patient who had undergone a kidney transplant and, in fact, was under immunosuppressant therapy. The nephrologist assessed that with the proper titration of the medication, this patient could be taken up for surgery without much risk for transplant rejection.
In addition, post total parotidectomy pathology reported the invasion of lymphovascular structures. These prove the presence of cancer cells both in lymphatic and blood vessels, pointing to a more tendency of risk for metastasis (Figure 5). It also found an extra-nodal extension. This bores the indication of extracapsular spread of the cancer cells into the peri glandular tissues, which may suggest that the increase in the risk of local recurrence.